The Pfizer Presentation That Got Dr. Robert Malone Kicked Off Twitter
Pfizer's own vaccine trial data shows an INCREASED risk of illness and death for the vaccinated.
STORY AT-A-GLANCE
Dr. Robert Malone says he was kicked off twitter for this 40-minute video presentation and PDF created by the Canadian Covid Care Alliance, which consists of over 500 independent doctors, scientists, and health care practitioners.
The video and PDF are a deep-dive into Pfizer’s own vaccine trial data which conclusively shows an INCREASED risk of illness and death for the vaccinated group compared to the placebo group. For example, there were 20 total deaths in the vaccinated group versus 14 total deaths in the placebo group with nearly double the amount of cardiovascular deaths among the vaccinated.
Furthermore, Pfizer unblinded the trial participants, tested the Covid-19 vaccine on healthy and young individuals rather than the sick and elderly and did not track any subclinical biomarkers which would be valuable early warning signs of disease caused by the vaccines.
Pfizer reported a 95% efficacy, which sounds like it protects you 95% of the time, but that 95% actually refers to the Relative Risk Reduction, while the Absolute Risk Reduction was only 0.84%. Moreover, the Pfizer clinical results are unreliable because Pfizer introduced immense subjectivity into the trial by leaving it up to the discretion of investigators whether or not to test participants for covid-19. There were a total of 3,410 participants who had Covid-19 symptoms but were not tested for Covid-19.
Pfizer reported one of their 12-year-old trial participants who has been paralyzed to a wheelchair and forced to eat from a feeding tube for the last 10-months as “functional abdominal pain”. Another whistleblower who was a regional director of Pfizer’s clinical trials also reported to the FDA that Pfizer had falsified data, unblinded participants, and not followed up on testing participants who reported symptoms, but neither the FDA nor Pfizer ever investigated the issue.
Pfizer’s report authors had conflicts of interest with 84% of them either employed by Pfizer, owning Pfizer stock, receiving grants from Pfizer, hired as consultants by Pfizer, or previously running clinical trials for Pfizer. For example, two of the Pfizer report authors actually made $9B in stock market profit directly from the Pfizer vaccines because they also happened to be the co-founders of BioNTech.
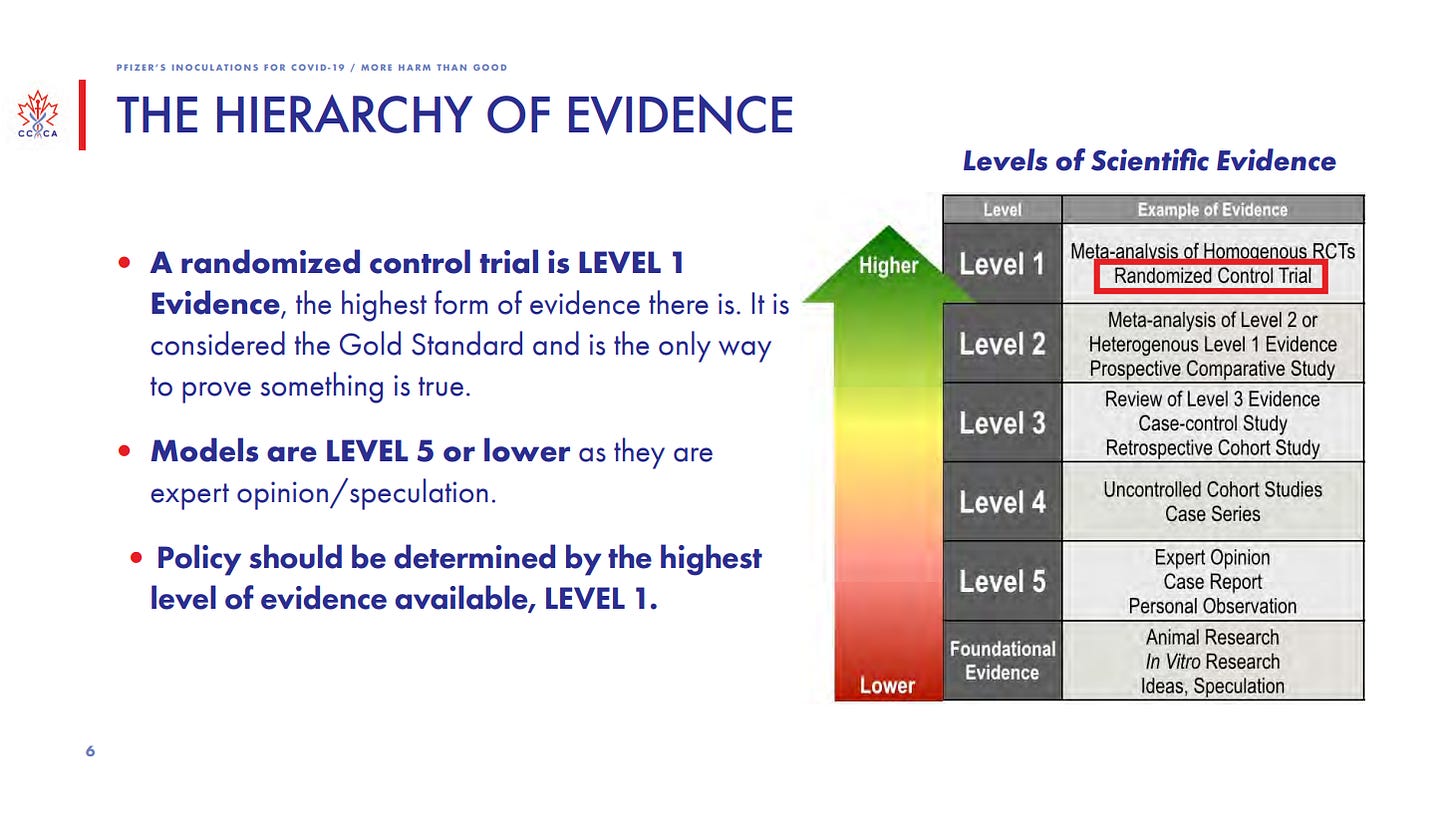
The Hierarchy of Scientific Evidence
When you are talking about proving things to be either safe or harmful, you need to rely on the best evidence. As you can see from the table below, a randomized controlled trial is considered the gold standard or level 1 evidence. This is the highest form of evidence there is and the only way to prove something is true. Models, which we have heard a lot of during the pandemic are actually the lowest form of evidence, level 5 or lower, as they are considered to be expert opinion or speculation. The policy should always be determined by the highest level of evidence available which is level 1.
Relative Risk Reduction vs. Absolute Risk Reduction
Pfizer reported that its vaccine shows a 95% efficacy. That sounds like it protects you 95% of the time, right? But that’s not actually what that number means. That 95% refers to the Relative Risk Reduction, but it doesn’t tell you how much your overall risk is reduced by vaccination. For that, we need Absolute Risk Reduction. In the Pfizer trial, 8 out of 18,198 people who were given the vaccine developed COVID-19. In the unvaccinated placebo group 162 people got it, which means that even without the vaccine, the risk of contracting COVID-19 was extremely low at 0.88%, which the vaccine then reduced to 0.04%. So the net benefit or the Absolute Risk Reduction that you’re being offered with a Pfizer vaccine is 0.84%. That 95% number refers to the relative difference between 0.88% (unvaccinated) and 0.04% (vaccinated) (0.88 – 0.04). That’s what they call 95% Relative Risk Reduction. Relative Risk Reduction is well known to be a misleading number, which is why the FDA recommends using Absolute Risk Reduction instead, which begs the question of how many people would have chosen to take the COVID-19 vaccines had they understood that they offered less than 1% benefit?
Pfizer Unblinded The Study After Two Months
There was an inoculated group and a placebo group of about 21,000 participants each, and they began the phase three trials in July of 2020. The study was blind, which means the participants didn’t know which group they were in. This blinded trial was supposed to go on for three years until May 2, 2023, and that would mark the end of phase three of the clinical trial. Instead, after only two months of trial data, Pfizer unblinded the study. This means they told all of the placebo and inoculation group participants which group they were in and offered the placebo group participants the option of moving over to the inoculated group. Most of them took Pfizer up on that offer, and the vast majority of the placebo group moved into the inoculated group. Therefore, after two months, there was no longer a control group to compare the inoculated group to and there was no longer any way to assess the long-term safety or effectiveness.
The Vaccinated Group Showed An Increased Risk of Illness
Pfizer’s data before the trial was unblinded shows a significant increase in the risk of illness for the vaccinated group versus the placebo group. You won’t actually find it in the report itself. You have to dig into the supplementary appendix in order to find it. There you find, 5,241 related adverse advents in the vaccinated group versus 1,311 related adverse events in the placebo group. For severe adverse events, there were 262 in the vaccinated group versus 150 in the placebo group. And, for serious adverse events, there were 127 in the vaccinated group versus 116 in the placebo group. In summary, for the vaccinated group, there was a 300% increase of related adverse events, a 75% increase of severe adverse events, and a 10% increase of serious adverse events.
The Vaccinated Group Also Showed An Increased Risk of Death
Pfizer’s data also shows an increased risk of death for the vaccinated. Before the trial was unblinded, there were 15 deaths in the vaccinated group versus 14 deaths in the placebo group. After the trial was unblinded, there were 5 more deaths in the vaccinated group with 3 of those deaths from the original vaccinated group (before trial was unblinded) and 2 of those deaths from the original placebo group (crossed over to the vaccinated group). In summary, there were 20 total deaths in the vaccinated group versus 14 total deaths in the placebo group. In the vaccinated, 9 deaths were attributed to cardiovascular events while only 4 deaths were attributed to cardiovascular events in the placebo group.
Pfizer Did Not Follow Established Protocols
In normal circumstances, developing a vaccine takes a total of ten years. On rare occasions, vaccines can be developed in as little as five years. The covid-19 vaccines were developed in less than one year. In order to expedite the process, animal testing was skipped, phases two and three were combined into two months, emergency use was authorized, the trial was unblinded, and the rollout began. Despite the persistent claim that Covid-19 vaccines are safe and do not need to be tested, there are still a lot of safety concerns about the Covid-19 vaccines and there are still a lot of unknowns about the mRNA delivery mechanism.
Misleading Demographics Wrong Age For Target Population
Pfizer’s trial used a younger age group than those who are most at risk from covid-19. 85% of people most at risk of death from covid-19 are over the age of 75, yet only 4% of the Pfizer clinical trial participants were over the age of 75. Pfizer chose participants from a younger demographic that would be less likely to need a vaccine, less likely to suffer an adverse event, and more likely to respond well, as the elderly have comparatively poor immune responses.
Misleading Demographics Tested On Healthy, Given To Sick
While 95% of people who have died with covid-19 have had at least 1 co-morbidity with an average of 4 co-morbidities, only 21% of participants in Pfizer’s clinical trial had co-morbidities. Pfizer excluded a list of health conditions from the trial including pregnant or breastfeeding women, people with allergies, psychiatric conditions, immunocompromised people, people with bleeding disorders, people who previously tested positive for Covid-19, people who had been prescribed steroids, etc. Therefore, there is no data to make safety claims about people with health conditions that were excluded from the trial. The vaccines were tested on the healthy, and then immediately given to the frailest members of society - the elderly with multiple health conditions. This is unscientific and unethical.
Inadequate Control Groups Exclude Natural Immunity
Pfizer’s clinical trial only observed two groups: the unexposed & vaccinated and the unexposed & unvaccinated. Pfizer’s trial should have also observed two more groups; the exposed & vaccinated and the exposed & unvaccinated. This data would have shown if the vaccine was safe for people with natural immunity and how the vaccines compared to natural immunity. It is very telling that Pfizer did not want to compare the safety and efficacy of their vaccines to natural immunity.
Low Quality Safety Science Did Not Track Biomarkers
Pfizer’s clinical trial did not track biomarkers and did not test for adverse events at the subclinical (pre-symptom) level. This is extremely unsafe because symptoms & diseases are typically endpoints of processes that can take months, years, or decades to surface. High-quality safety science would have tested before and after inoculation for; d-dimers for evidence of enhanced coagulation, c-reactive protein for evidence of enhanced inflammation, troponins for evidence of cardiac damage, blood oxygen levels for evidence of enhanced hypoxia, amyloid-beta and phosphorylated tau for evidence of increased predisposition to Alzheimer’s disease, serum HMGB1, CXCL13, and Dickkopf-1 for evidence of an increased disposition to autoimmune disease, etc. Pfizer should have been tracking biomarkers that would have been early warning indicators for disease caused by the vaccines.
Clinical Endpoints Should Have Been All-Cause Illness & Mortality
Pfizer used the wrong clinical endpoints by focusing on the prevention of covid-19 rather than all-cause illness and mortality. All-cause illness and mortality should be the clinical endpoints rather than just illness and death with covid-19, in order to make sure that the vaccines are not causing harm. This was learned decades ago with cancer drug trials. For example, cancer drug trial clinical endpoints were switched to “all-cause illness and mortality” from “did the drug shrink the cancer?” because some drugs were not only killing cancer but were also killing patients.
Not Tested For Spread Reduction Vaccine Passports Unjustified
Vaccine passports are now being pushed to ostensibly prevent or reduce transmission of covid-19, but this outcome was never studied in the clinical trials and it is inappropriate to assign that capability to these vaccines. There is no evidence at all that they reduce the spread of covid-19 or transmission because this was never one of the clinical trial endpoints.
Testing Failures Subjective Testing
Pfizer introduced a high level of subjectivity by leaving it up to the discretion of investigators whether or not to test participants for Covid-19. This means the Pfizer trial completely missed asymptomatic infections. Furthermore, this lack of a systematic, objective approach to testing makes the entire results of the trial unreliable. All trial participants should have been regularly tested for Covid-19.
Missing Data Lost To Follow Up & Suspected But Unconfirmed
Pfizer did not test 3,410 suspected cases who were symptomatic with covid-19 because the discretion for testing was left up to the investigator. This included 1,594 participants in the vaccinated group and 1,816 participants in the placebo group. Pfizer also lost contact with 80 participants in the vaccinated group and 86 participants in the placebo group which means they were unable to confirm whether another 186 participants got sick or not. The fact that the number of subjects Pfizer lost contact with and the number of subjects symptomatic but never tested are significantly higher than the clinical trial endpoint numbers means that the entire Pfizer clinical trial is speculative and unreliable.
12-15 Adolescent Trial All Risk No Benefit
For the 12 to 15 adolescent trial, the study was severely underpowered to show the risk of adverse events. There was a vaccinated group of 1,005 (0 tested positive for covid-19) and a placebo group of 978 (18 tested positive for covid-19). Pfizer claimed these were great results, but since adolescents are at statistically 0% risk of death from covid-19, and very low risk of severe illness, the vaccination is of little benefit to them and only presents a very real risk of adverse events which Pfizer’s trial was not designed to find. But in this case, among the 1,005 adolescents, there was at least one serious adverse event - Maddie de Garay.
12-15 Adolescent Trial Failure To Report Serious Adverse Events
Maddie de Garay is a 12-year-old trial participant who developed a serious reaction after her second dose and was hospitalized within 24 hours. She developed gastroparesis, nausea and vomiting, erratic blood pressure, memory loss, brain fog, headaches, dizziness, fainting, seizures, verbal and motor tics, menstrual cycle issues, lost feeling from the waist down, lost bowel and bladder control, and she had to have a feeding tube because she lost her ability to eat. She has been hospitalized many times, and for the past 10 months, she has been wheelchair-bound and fed via a tube. In their report to the FDA, Pfizer described her injuries as “functional abdominal pain.” This is unconscionable and certainly opens up the possibility that other adverse events have been suppressed or misrepresented.
5-11-Year-Olds Risking Their Health
For 5 to 11-year-olds, these vaccinations are an unacceptable risk. In this table, Pfizer, uses predictive modeling to acknowledge that their vaccinations WILL cause myocarditis, but optimistically claim that there will be zero deaths from myocarditis. This is speculation on their part using the low-level evidence of a predictive model. But even if it were true, there is no justification for giving children myocarditis or causing any harm to children. First, do no harm, should apply here.
Myocarditis Is Serious
Myocarditis is very serious. It is damage to the heart and it is not reversible. Myocarditis is an inflammatory process of the myocardium (heart muscle). Severe myocarditis weakens your heart so that the rest of your body doesn't get enough blood. Clots can form in your heart, leading to a stroke or heart attack. The mortality rate for myocarditis is up to 20% at 6.5 years and this is unequivocally an unacceptable risk for children.
FDA Abandons First Do No Harm
The FDA has actually abandoned the first do no harm principle and says that this is an acceptable risk for children. Medical interventions are supposed to be proven safe before they are rolled out in the population. Yet Dr. Eric Rubin, one of the 18 members of the FDA advisory panel who voted, to approve the inoculations for children 5 - 11, actually said the opposite and suggested that a population level rollout was an appropriate way to test for adverse events. It’s worth noting that Dr. Eric Rubin is the editor-in-chief of the New England Journal of Medicine, which also published the Pfizer trial reports.
5-11-Year-Olds No Informed Consent
Direct-to-consumer advertising of prescription drugs is illegal in Canada, yet politicians from all levels of government are marketing inoculations to children, using cartoons and mascots. They are proclaiming the vaccines to be safe, yet the data is not there to back that up. In addition to admitting that their inoculations can cause myocarditis, Pfizer also admits, right in their report, that their long-term immune response, efficacy & safety data is limited and that their studies were not powered to find “rare” side effects as only 1,517 kids got the inoculations. How many parents would take their kids to get this shot if they were informed of this? The law of informed consent says they should be, but it is not happening.
British Medical Journal Publishes Whistleblower Story
On November 2nd, the British Medical Journal released an article about their investigation into Ventavia, one of the research companies Pfizer hired to conduct the trials. The whistleblower is a Regional Director who actually reported her company to the FDA for falsifying data, unblinding participants, not following up and testing participants who reported symptoms and mislabeling specimens. Several other employees backed up her account. Despite all this, neither Pfizer, nor the FDA ever audited or investigated the research company, Pfizer never disclosed the problems in its EUA application, and in fact, Pfizer has now hired that same researcher, Ventavia, to run four more Covid-19 clinical trials.
Roll Out Surveillance You Don’t Find What You Don’t Look For
Governments are assuring us that they will monitor the vaccine roll-out closely and if there are problems then they will find them. But is that true? If you look at the two charts below, the one on the left represents active surveillance and the one of the right represents passive surveillance. There is a dramatic difference between passive versus active monitoring of adverse events. The first bar on the left represents participants in the Pfizer trial given an app and asked to chose from a list of adverse events they were experiencing during the first seven days after vaccination. 78% of participants reported an adverse event and 5% reported a severe adverse event. The chart on the right shows how much the signal is lost through passive surveillance systems like Health Canada, CDC VAERS, or the European Yellow Card system. It is not reasonable to believe that a vaccine that elicited adverse events in 78% of trial subjects elicited essentially 0% adverse events in a population wide rollout. What is happening is the signal has been lost. It is not that the adverse events are gone, instead we are not finding them because we are not looking for them.
Rising Incidents of Heart Issues In Young People
Some signals are getting through as we are hearing more and more about rising incidents of heart issues in young people. Ontario Public Health is well aware of this, as they published a report on it, but they seem inconsistent in their concerns. On September 29, 2021, Ontario Public Health recommended young men 18-24 not take the Moderna shot, because of a 1 in 5,000 risk of myocarditis. They suggested Pfizer shot instead, which has a 1 in 28,000 risk of myocarditis. But as recently as May 8, 2021, Ontario had stopped the Astra Zeneca shot because of a 1 in 60,000 risk of clotting side effects, which was considered too high. Their priorities are inconsistent.
This Is Not Normal
All of this is leading to a lot more deaths in young people than is normal and it seems to be especially showing up in athletes who really push their heartrates up when they are exercising. A German news site put together a list of over 75 known cases of athletes collapsing - and even dying - in the last 5 months. An Israeli news site analyzed the number of sudden deaths “on the pitch” of members of the International Football Association (FIFA) over the past 20 years. The average number of FIFA sudden deaths between 2000 - 2020 was 4.2. In 2021, it was 21 or five times the normal average.
This Is Supposed To Be Rare
Pfizer’s Post Marketing Pharmacovigilance Report
On Nov 17, 2021, the FDA released the first batch of what will ultimately be 329,000 pages they were ordered by a court to provide to satisfy a Freedom of Information request by a group called Public Health and Medical Professionals for Transparency who want access to the data used by the FDA to approve Pfizer’s COVID-19 inoculations. One post-marketing pharmacovigilance report submitted to the FDA, where Pfizer tracked real-world adverse events occurring in the first 2.5 months after Emergency Use Authorization, was particularly disturbing. There were over 1,200 deaths, over 25,000 nervous system adverse events, and under “safety concerns” Pfizer listed Anaphylaxis and Vaccine-Associated Enhanced Disease. This document should be incriminating for any agency who saw it and called these inoculations “safe.”
Pfizer Is Making Billions Despite Criminal History
Pfizer is making more than $33.5B from their covid-19 vaccines in 2021 alone. Their agenda is their shareholders and their bottom line is not public health. Over the years, Pfizer has been found to have engaged in many criminal activities including lying to get Federal approval for a heart valve that fractured and killed hundreds of patients worldwide, conducting clinical trials on African children without their parent’s consent after which some of the children died, bribing doctors, suppressing research, manipulating studies, withholding information that their products caused cancer, fraudulent marketing, and many more egregious crimes. They have paid billions in fines and settlements for their repeated criminal actions over the last few decades.
Conflicts Of Interest Among Pfizer Report Authors
There are massive conflicts of interest among the Pfizer report authors. For example, for the six-month report authors, 84% of the authors have conflicts of interest. They were either employed by Pfizer, own stock in Pfizer, have gotten grants from Pfizer, have been hired as consultants by Pfizer, or have run clinical trials for Pfizer. There were only 5 authors that did not have conflicts of interest with Pfizer and none of them are any of the main authors. The leading author, the corresponding author, and the last author all have conflicts of interest. The most eye-popping example of conflict is the two founders of BioNTech are both authors of the Pfizer six-month trial report. These two alone profited $9B in stock value from the Pfizer inoculations, yet they are still allowed to author the Pfizer reports.
The CDC Has Redefined “Vaccine”
The CDC redefined “vaccine” to suit political and pharmaceutical interests. For many years, the CDC definition of “vaccine” was "a product that stimulates a person’s immune system to produce immunity to a specific disease, protecting the person from that disease." Starting on September 2, 2021, the CDC redefined “vaccine” to "a preparation that is used to stimulate the body’s immune response against diseases." Pfizer’s Covid-19 vaccines did not fit the CDC’s original definition of vaccine so the CDC changed the definition of “vaccine”.
A lot of people can not believe that Pfizer’s clinical trial data could be so fundamentally flawed and even show an increased risk of illness and death for the vaccinated. They think that if this were true, wouldn’t the media be reporting the truth about Pfizer’s vaccines? We think that this video answers that question:
It’s clear that the inoculations should be withdrawn immediately. It’s clear that Pfizer - and the agencies overseeing their trials - failed to follow established, high-quality safety and efficacy protocols right from the beginning. We have presented Level 1 evidence of harm from Pfizer’s own trial data.
Any government which has approved these inoculations much less mandated them, knew or should have known from the available data that harm would be caused to its citizens. Any government that approved this medical intervention for its citizens should have ensured that the trial had used the appropriate clinical endpoints and high-quality safety science. Any government official who possesses this evidence and continues to allow its citizens to be inoculated with a toxic agent is, at the very least, negligent.
We need YOU to hold them accountable.
This evidence is a tool you can use. It represents a real opportunity to hold our leaders accountable as it is not opinion, or modeling, or real-world evidence that can be dismissed or manipulated, but LEVEL 1 EVIDENCE from a randomized control trial. We’re asking that you call your politicians and that you ask for a 1-hour meeting.
During the meeting, play them the video and provide them with the PDF version. Ask them questions, like whether or not they were aware of all the issues with the Pfizer trial. Or what they plan to do now that they are. Get them to agree to a follow-up meeting where they will provide you with answers.
Share this article on social media. Share this article with family and friends. Thank you in advance for spreading this information far and wide. We cannot make a change unless we all work together.
You can also find me on telegram, rumble, and gab.

































The most comprehensive article I’ve found so far.
Well done!! Sharing far and wide.
Just stunning what has been kept hidden.
Keep up your stellar work!!!! Mahalo!!🙏🏽❤️🌺